MUPS is a multi particulate pharmaceutical solid dosage form produced by
compressing a mixture of drug-containing pellets and powder Excipients. The
pellets have a spherical core that contains or is coated with the active
ingredient, and have one or more protective layers (cellulosic and acrylic polymers) to control drug release.
MUPS technology has been adopted by the pharmaceutical industry as an
alternative to conventional immediate or modified release tablets. Offering
increased bioavailability and improved pharmacological properties, including
sustained release, enteric-coated pellets containing different drugs, and
subsequently tablet, can be used to protect the API from gastric media.
Compressing pellets reduces the esophageal residence time, compared with
capsules and improves physicochemical stability.
Further, compared with other delivery systems, MUPS formulations offer a
lower risk of local irritation and toxicity, reduced dose dumping, minimal
plasma concentration fluctuations and the ability to administer high potency
products. More reproducible pharmacokinetic behavior and lower
intra/inter-subject variability compared with conventional formulations have
also been reported. Other benefits include taste masking and controlled
absorption.
MUPS
Formulation Factors
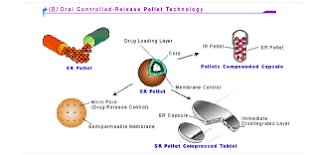
Pellets that are included in MUPS tablets can be uncoated or coated. The drug may be included in the core or as a layer applied to the inert core of the pellet. In addition, the pellets may have one or more layers that may include suitable Excipients for modified release such as polymers for enteric coating or polymers for sustained release. Uncoated pellets are made of suitable pharmaceutical Excipients such as lactose and microcrystalline cellulose (MCC), among others.
Pellets that are included in MUPS tablets can be uncoated or coated. The drug may be included in the core or as a layer applied to the inert core of the pellet. In addition, the pellets may have one or more layers that may include suitable Excipients for modified release such as polymers for enteric coating or polymers for sustained release. Uncoated pellets are made of suitable pharmaceutical Excipients such as lactose and microcrystalline cellulose (MCC), among others.
Coated
pellets must be produced with the appropriate polymer and amount to form the
coating film. In addition, the stability of the pellet's coating film depends
on compression forces applied.
Most
studied polymers used to create the pellet's coating film are cellulosic and
acrylic polymers. The advantages of acrylic polymers are the flexibility and
adequate features that enable the tablet process without rupturing of the
pellet's coating film. Combining two types of these polymers improve the
coating film flexibility which is desirable for coated pellets elaboration, as
well as the addition of a plasticizer in a certain proportion [3].
Another
important issue that influences drug release from MUPS is the pellet core.
Pellets porosity of both, uncoated and coated pellets, affect the modified drug
release profile. Pellet core design should be carefully studied previous to
formulation.
The Excipients
and binder liquid used to produce the pellet core may affect the deformation
and viscoelastic properties of the pellet during compression, and thus cause
changes in drug release profile [1]. The use of other components like carrageen
an polysaccharide in the production / manufacture of the pellets, allows them
to have a rapid disintegration and therefore a fast drug release.
MUPS
Manufacturing Process
The manufacturing process of these tablets is tough and constitutes a challenge when considering all the steps involved in the process.
The manufacturing process of coated pellets can be divided into two steps, pellet manufacture and tablets containing pellets manufacture. In fig. 1, both processes are described. First, the drug-pellet manufacturing process begins with the blending of pellet components such as drug, cushioning Excipients like microcrystalline cellulose, Glycerin Monostearate (GMS) and Lactose Monohydrate (LM) which are widely used in this kind of formulation. A binder liquid must be used (water or glycerol) for wet mixing. The mass obtained continues through the extrusion-spheronisation process and the drying of pellets recently formed can be performed in a fluid bed dryer. The next step, pellet coating, requires a careful selection of the Excipients that will form the coating film in order to obtain the desired drug release [6].
The manufacturing process of these tablets is tough and constitutes a challenge when considering all the steps involved in the process.
The manufacturing process of coated pellets can be divided into two steps, pellet manufacture and tablets containing pellets manufacture. In fig. 1, both processes are described. First, the drug-pellet manufacturing process begins with the blending of pellet components such as drug, cushioning Excipients like microcrystalline cellulose, Glycerin Monostearate (GMS) and Lactose Monohydrate (LM) which are widely used in this kind of formulation. A binder liquid must be used (water or glycerol) for wet mixing. The mass obtained continues through the extrusion-spheronisation process and the drying of pellets recently formed can be performed in a fluid bed dryer. The next step, pellet coating, requires a careful selection of the Excipients that will form the coating film in order to obtain the desired drug release [6].
The
tablet process will be performed by a rotary tablet press machine and tablet
parameters like main compression force or speed constitute important issues
that will be mentioned later. Pellets and cushioning Excipients will be added
for tablet, taking into account the Excipients' adequate properties in order to
withstand high compression forces.
Tablets
containing pellets with specific features of shape, weight, thickness, and
hardness then continue through the tablet film coating process. The tablet film
coating is applied to improve the stability and appearance of the
pharmaceutical composition, and has no significant influence on drug release
upon tablet disintegration, as it is one of the functions of pellet coating
film.
One of
the main problems observed in the manufacturing of modified release MUPS is
that the compaction process of pellets to obtain the tablet can cause the
breakdown of the polymer coat and alters the mechanical properties and release
rate of the drug from the pellets. Therefore, the optimization of the pellet
formulation to prevent pellet deformation during compaction may be critical. To
overcome this problem, cushioning agents, such as polyethylene glycol among
others are commonly used.
Drug
Release
Usually the multiple unit pellet systems (MUPS) are designed to obtain a modified release profile of drugs. This modified release should be considered as delayed release or sustained release. The delayed release can be achieved, for example, by enteric-coated pellets. Enteric-coating allows that active pharmaceutical ingredients that are unstable in gastric media or may cause gastric irritation are protected by an enteric coating. Methacrylic acid copolymers, Hydroxypropyl methylcellulose phthalate, and Hydroxypropyl methylcellulose acetate succinate are enteric-coating polymers frequently used for this function.
Usually the multiple unit pellet systems (MUPS) are designed to obtain a modified release profile of drugs. This modified release should be considered as delayed release or sustained release. The delayed release can be achieved, for example, by enteric-coated pellets. Enteric-coating allows that active pharmaceutical ingredients that are unstable in gastric media or may cause gastric irritation are protected by an enteric coating. Methacrylic acid copolymers, Hydroxypropyl methylcellulose phthalate, and Hydroxypropyl methylcellulose acetate succinate are enteric-coating polymers frequently used for this function.
MUPS
tablets containing sustained release pellets will achieve sustained action and
prolong the pharmacological effect, extend the dosage interval and reduce side
effects.
The
sustained release can be achieved through different strategies such as:
1. The use of pellets coated with different polymers and different film thicknesses that allow modulation of the release rate from pellets. The polymers used can be, among others, cellulose derivatives such as ethyl cellulose and hydroxypropyl methylcellulose (HPMC).
1. The use of pellets coated with different polymers and different film thicknesses that allow modulation of the release rate from pellets. The polymers used can be, among others, cellulose derivatives such as ethyl cellulose and hydroxypropyl methylcellulose (HPMC).
2. The
use of uncoated pellets as a matrix polymeric system for sustained release of
the drug. In this group the hydrophilic matrix systems based on the use of cellulosic
polymers, carbomers or xantham gums, among others are frequently used. Numerous
factors related to the physicochemical characteristics of the drug, type,
percentage and other characteristics of the polymer and formulation factors can
affect the release rate from these systems [9,10].
Figure 2
shows the biopharmaceutic and pharmacokinetic behavior of MUPS tablets for
modified release in the gastrointestinal tract.
MUPS
Applications
The MUPS technology is used successfully as an alternative to conventional tablets. Enteric coated pellets containing different drugs and packed into tablets are commonly used to protect drugs from gastric media. It has been shown that Omeprazole MUPS have increased bioavailability and improved pharmacological response.
The MUPS technology is used successfully as an alternative to conventional tablets. Enteric coated pellets containing different drugs and packed into tablets are commonly used to protect drugs from gastric media. It has been shown that Omeprazole MUPS have increased bioavailability and improved pharmacological response.
Another
important application of the tablet is in the field of sustained release with
different drugs such as diclofenac, theophylline, metoprolol and pseudoephedrine
among others.
In
conclusion these tablets are an interesting alternative for modified drug
release for oral administration.